Institutional News
Dr. Hui Jiang’s lab identified a new pathway mediating mitochondrial outer membrane protein degradation
On Jan 2, 2018, Hui Jiang’s lab at National Institute of Biological Sciences (NIBS), Beijing, published a paper titled “Mitochondrial inner-membrane protease Yme1 degrades outer-membrane proteins Tom22 and Om45” on Journal of Cell Biology.
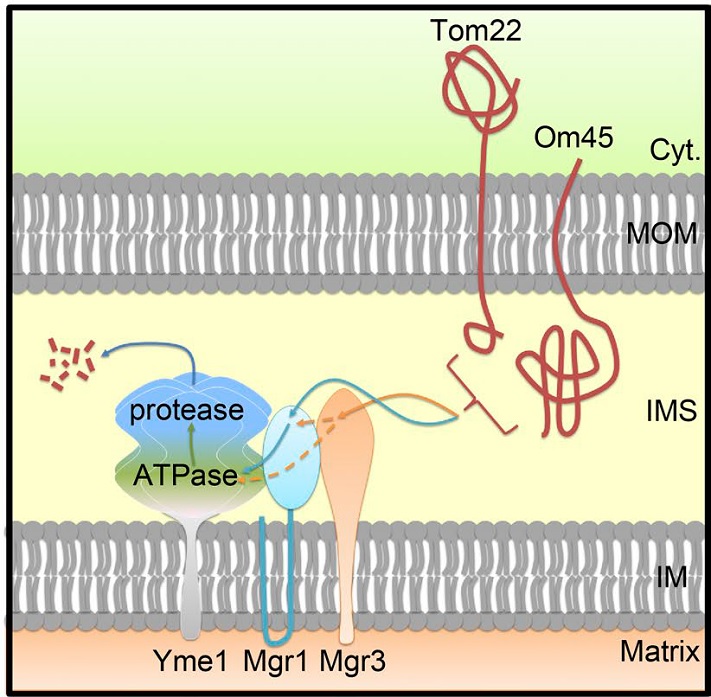
Mitochondria are double-membraned organelles playing essential metabolic and signaling functions. The mitochondrial proteome is under surveillance by two proteolysis systems: the ubiquitin–proteasome system degrades mitochondrial outer-membrane (MOM) proteins, and the AAA proteases maintain the proteostasis of intramitochondrial compartments. Jiang lab previously identified a Doa1–Cdc48-Ufd1-Npl4 complex that retrogradely translocates ubiquitinated MOM proteins to the cytoplasm for degradation. In this study, they report the unexpected identification of MOM proteins whose degradation requires the Yme1-Mgr1-Mgr3 i-AAA protease complex in mitochondrial inner membrane. Through immunoprecipitation and in vivo site-specific photo–cross-linking experiments, the authors show that both Yme1 adapters Mgr1 and Mgr3 recognize the intermembrane space (IMS) domains of the MOM substrates and facilitate their recruitment to Yme1 for proteolysis. The authors also provide evidence that the cytoplasmic domain of substrate can be dislocated into IMS by the ATPase activity of Yme1. These findings indicate a proteolysis pathway monitoring MOM proteins from the IMS side and suggest that the MOM proteome is surveilled by mitochondrial and cytoplasmic quality control machineries in parallel.
Dr. Xi Wu is
the first author of this paper. Lanlan Li is the second author. Xi Wu and Hui
Jiang are corresponding authors. The work was supported by grants from the
Ministry of Science and Technology of China, Municipal Government of Beijing,
China Postdoctoral Science Foundation and Beijing Postdoctoral Research Foundation.



