Research News
Tao Wang’s laboratory identified a novel ERAD pathway
On Nov. 2nd 2020, Dr. Tao Wang's laboratory identified two novel E3 ubiquitin ligases SORDD1 and SORDD2 representing a new ER-associated protein degradation (ERAD) pathway. The work entitled “Suppression of retinal degeneration by two novel ERAD ubiquitin E3 ligases SORDD1/2 in Drosophila.” is published online in the Plos Genetics (doi: 10.1371/journal.pgen.1009172).
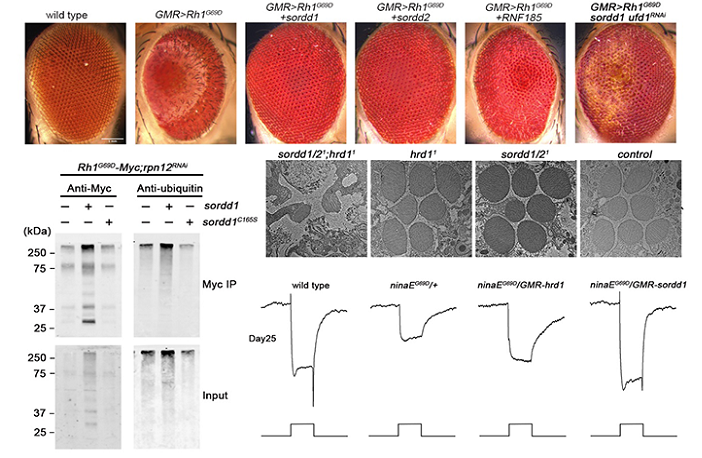
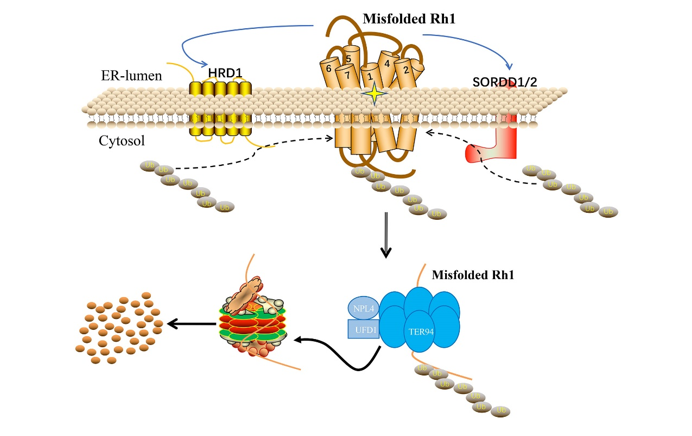
A large number of human diseases including autosomal dominant retinitis pigmentosa (adRP) are thought to reflect misfolding and/or ER-retention of membrane proteins. Proteasomal degradation of misfolded proteins through ERAD efficiently reduces their accumulation, and thus plays a key protective role. In this article, the authors established of adRP by expression of a misfolded and ER-accumulated mutant form of the major rhodopsin, Rh1G69D, and conducted a genome-wide gain of function screen for modifiers of this retinal cell death. As results the authors identified highly homologous ER resident RING domain E3 ubiquitin ligases, named as SORDD1 (Suppression Of Retinal Degeneration Disease 1 upon overexpression) and SORDD2, which strongly suppressed retinal degeneration in Rh1G69D-expressing flies. SORDD1/2 transferred poly-ubiquitin chains to misfolded Rh1 in vivo, promoting the degradation of mutant Rh1 protein, thereby suppressing neurodegeneration. A subsequent RNAi screen for factors required for SORDD1-mediated degradation of Rh1G69D identified the VCP complex and the proteasome are required for SORDD1/2’s function. Moreover, SORDD1/2 promoted the degradation of Rh1G69D independent of the classic HRD1/HRD3 complex (E3 ubiquitin ligase and retro-translocation channel in ERAD). Instead, SORDD1/2 and HRD1 functioned in parallel to degrade misfolded rhodopsin, and maintain photoreceptor cell function and integrity, as double mutations of sorrd1/2 and hrd1 caused dysfunction and degeneration of photoreceptor neurons. Finally, the human homologues of SORDD1/2 also suppressed Rh1G69D-mediated retinal degeneration. These findings identify a novel ERAD pathway that acts in parallel to HRD1, and reveal novel therapeutic targets for treating adRP.
Jiawei Xu, a PhD student from China Agricultural University is the first author of this article, Drs. Haifang Zhao and Tao Wang are the co-authors. The study was supported by the National Natural Science Foundation of China and the Chinese Ministry of Science. The research was conducted at the National Institute of Biological Sciences, Beijing.



